2018
Branched copolymer-stabilised nanoemulsions as new candidate oral drug delivery systems
J. J. Hobson, S. Edwards, R. A. Slater, P. Martin, A. Owen & S P. Rannard
RSC Advances, 2018, 8, 12984 - 12991
Assessment of interactions of efavirenz solid drug nanoparticles with human immunological and haematological systems
N. J. Liptrott, M. Giardiello, T. O. McDonald, S. P. Rannard & A. Owen
Journal of Nanobiotechnology, 2018, 16, 22
Towards a Maraviroc Long-Acting Injectable Nanoformulation
L.M. Tatham, A. C. Savage, A. Dwyer, M. Siccardi, T. Scott, M. Vourvahis, A. Clarke, S. P. Rannard & A. Owen.
European Journal of Pharmaceutics and Biopharmaceutics, 2018, S0939-6411(18)30200-5
Modulated release from implantable ocular silicone oil tamponade drug reservoirs.
H. Cauldbeck, M. Le Hellaye, T. O. McDonald, M. Long, R. L. Williams, S. P. Rannard and V. R. Kearns
J. Polym. Sci., Part A: Poly. Chem., 2018, 56, 938–946
Co-initiated hyperbranched-polydendron building blocks for the direct nanoprecipitation of dendron-directed patchy particles with heterogeneous surface functionality.
F. Y. Hern, A. Hill, A. Owen and S. Rannard
Polym. Chem., 2018, 9, 1767 - 1771
Long-acting injectable atovaquone nanomedicines for malaria prophylaxis.
R. P. Bakshi, L. Tatham, A. C. Savage, A. K. Tripathi, G. Mlambo, M. M. Ippolito, E. Nenortas, S. P. Rannard, A. Owen, T. A. Shapiro
Nature Communications, 2018, 9, 315
Improving maraviroc oral bioavailability by formation of solid drug nanoparticles
A.C. Savage, L.M. Tatham, M. Siccardi, A. Owen, T. Scott, M. Vourvahis, A. Clark & S.P. Rannard
European Journal of Pharmaceutics and Biopharmaceutics, 2018, S0939-6411(18)30165-6
In Silico Dose Prediction for Long-Acting Rilpivirine and Cabotegravir Administration to Children and Adolescents
R.K.R. Rajoli, D.J. Back, A. Owen, M. Siccardi,
S.P. Rannard, C. F. Meyers & C. Flexner
Clinical Pharmacokinetics 2018, 57, 255-266
Recommendations for clinical translation of nanoparticle-enhanced radiotherapy
K. Ricketts, R. Ahmad, L. Beaton, B. Cousins, K. Critchley, M. Davies, S. Evans, I. Fenuyi, A. Gavriilidis, Q. J. Harmer, D. Jayne, M. Jefford, M. Loizidou, A. Macrobert, S. Moorcroft, I.Naasani, Z. Y. Ong, K. M Prise, S. Rannard, T. Richards, G. Schettino, R. A. Sharma, O. Tillement, G. Wakefield, N. R. Williams, E. Yaghini & G. Royle
The British Journal of Radiology 2018, 19, 20180325
The emerging role of physiologically based pharmacokinetic modelling in solid drug nanoparticle translation
M. Siccardi, S. Rannard & A. Owen
Advanced Drug Delivery Reviews 2018, 131, 116-121.
Inhibitory Effects of Commonly Used Excipients on P-Glycoprotein in Vitro
R. Gurjar , C. Y. S. Chan, P. Curley, J. Sharp, J. Chiong,
S. Rannard, M. Siccardi & A. Owen
Mol. Pharmaceutics, 2018, 15, 4835–4842
The potential value of nanomedicine and novel oral dosage forms in the treatment of HIV
J. J Hobson, A. Owen & S. P Rannard
Nanomedicine (London), 2018, 13, 1963-1965
2017
In Situ Xanthate Deprotection to Generate Thiol Chain Transfer Agents for Conventional Free Radical Linear and Branched Vinyl Polymerisation
S. Flynn, S. D. Dale, A. B. Dwyer, P. Chambon & S. P. Rannard
J. Polym. Sci., Part A: Poly. Chem., 2017, 55, 3963-3967
In vitro characterisation of solid drug nanoparticle compositions of efavirenz in a brain endothelium cell line
P. Curley, M. Giardiello, N. J. Liptrott, D. Dickens, D. M. Moss, J. Hobson, A. C. Savage, T. O. Mcdonald, M. Siccardi, S. Rannard & A. Owen
JOIN, 2017, 2, 157-169
Intracellular delivery of nano-formulated anti-tuberculosis drugs enhances bactericidal activity
S. Donnellan, V. Stone, C. Watkins, M. Gardiello, S. Rannard, A. Owen, H. Johnston, B. Swift, G. Aljayyoussi, J. McLuckie & K. Stevenson
JOIN, 2017, 2, 146-156
Lack of interaction of lopinavir solid drug nanoparticles with cells of the immune system
N. J. Liptrott, M. Giardiello, T. O. McDonald, S. P. Rannard & A. Owen
Nanomedicine, 2017,12, 2043-2054
MADIX polymerization of vinyl acetate using ethyl acetate as a green solvent; near-complete monomer conversion with molecular weight control
M. E. Levere, P. Chambon, S. P. Rannard &
T. O. McDonald
J. Polym. Sci., Part A: Poly. Chem., 2017, 55, 2427–2431
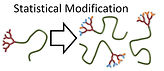
Model studies of the sequential and simultaneous statistical modification of dendritic functional groups and their implications within complex polymer architecture synthesis
F. Y. Hern, S. E. R. Auty, O. C. J. Andrén, M. Malkoch & S. P. Rannard
Polym. Chem., 2017, 8, 1644-1653
Simulating intestinal transporter and enzyme activity in a physiologically based pharmacokinetic model for tenofovir disoproxil fumarate
D. M. Moss, P. Domanico, M. Watkins, S. Park, R. Randolph, S. Wring, R. K. R. Rajoli, J. Hobson, S. Rannard, M. Siccardi & A. Owen
Antimicrob. Agents and Chemother., 2017, 61, e00105-17/1-e00105-17/9